Mozambique buys Kenyan-made HIV drugs, marking key step in Africa’s push to self-sufficiency
- Global Fund is procuring Kenyan-made HIV drugs for patients in Mozambique, making it the first time African-manufactured drug (TLD) has been deployed through this channel.
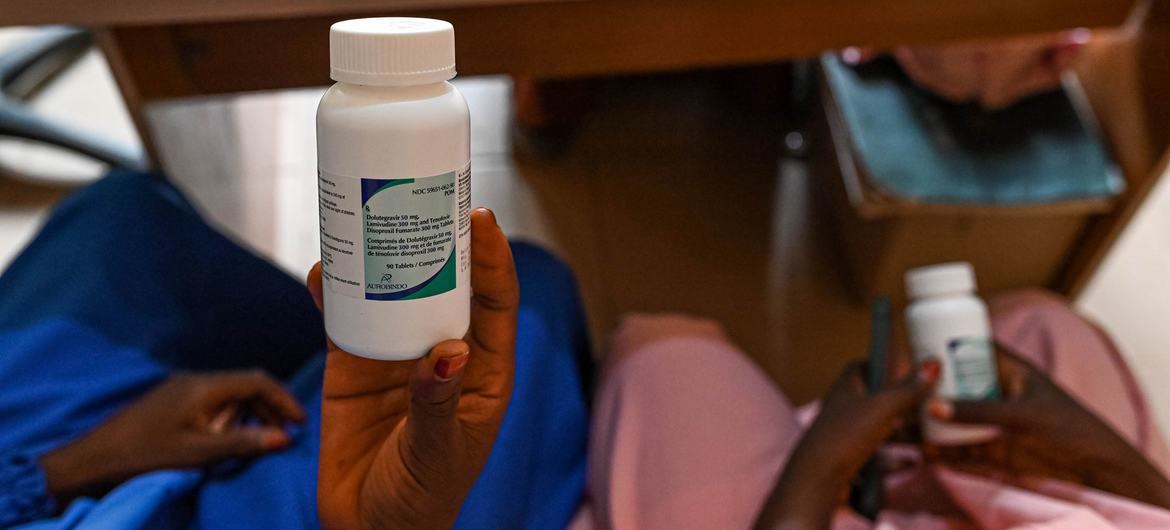
- In 2023, Kenya-based pharma Universal Corporation Ltd became the first African manufacturer to receive WHO nod to produce tenofovir disoproxil fumarate, lamivudine and dolutegravir (TLD) – a first-line antiretroviral therapy for HIV.
Africa’s self-reliance on the manufacture of life-saving HIV drugs has entered a new era with a first following the procurement of ‘Made in Africa’ medication by Mozambique from a manufacturer in Kenya.
In an update, the Global Fund – a worldwide alliance financing HIV, tuberculosis and malaria management programes said it is procuring Kenyan-made HIV treatment for patients in Mozambique, making it the first time African-manufactured drug (TLD) has been deployed through this channel.
“The procurement of the African-manufactured first-line HIV treatment by the Global Fund for Mozambique is a great milestone towards strengthening supply chain systems in Africa,” said Meg Doherty, Director of WHO’s Global HIV Programmes.
“This will contribute to better health outcomes for people living with HIV who need uninterrupted medicine supplies.”
Two years ago, Kenya-based pharmaceutical company Universal Corporation Ltd became the first African manufacturer to receive World Health Organization (WHO) prequalification to produce tenofovir disoproxil fumarate, lamivudine and dolutegravir (TLD) – a first-line antiretroviral therapy for HIV.
This step marks a major milestone in the fight against HIV and AIDs in Africa, a continent that accounts for over 65 percent of the disease burden internationally.
Building African capacity to make HIV drugs
According to the WHO, the establishment of Kenya-based pharmaceutical company, Universal Corporation Ltd, is part of a broader plan to enhance the production capacity of essential HIV drugs in Africa while also improving access to essential health technologies in the continent.
For years, the WHO has been working closely with authorities across economies in Africa, manufacturers and like-minded global health organizations such as the Global Fund and Unitaid – to significantly enhance quality-assured manufacturing based in Africa.
“Local production of quality-assured health products is an urgent priority,” said Rogerio Gaspar, WHO Director for Regulation and Prequalification.
“With every African manufacturer that meets WHO prequalification standards, we move closer to a more self-reliant, resilient and equitable health system.”
The human immunodeficiency virus (HIV) weakens the body’s immune system, significantly lowering its ability to fight infections and certain cancers.
Empirical evidence shows that without timely intervention, HIV can advance rapidly, turning to acquired immunodeficiency syndrome (AIDS), which is the most advanced stage of infection.
Africa still facing huge gaps in fighting disease
Even with Kenya-based HIV drug manufacturing capacity, WHO says that production alone is not enough. There is urgent need to ensure long-term sustainability of the drug making company, a goal that can only be achieved through sustained market commitments, deployment of fair procurement policies and ongoing technical support.
Additionally, WHO laments that many countries are still grappling with huge diagnostics challenges. And with thinning donor support in the near term, a huge number of countries stricken by the HIV and AIDs pandemic may struggle a lot to maintain reliable HIV testing programmes for millions of people at risk of infection, let alone sustain robust prevention and treatment systems.
Nigeria steps up HIV drugs making capacity
Also encouraging is a recent development in Nigeria where a diagnostics company, Codix Bio, received a sublicense to manufacture rapid diagnostic tests for HIV.
“Having locally produced HIV rapid tests will help increase affordability, and more broadly address supply chain vulnerabilities and delays in access to diagnostics,” said Dr. Doherty.
As part of its guidance, WHO is encouraging countries to adopt low-cost, WHO-prequalified rapid HIV tests, especially as the first test in national algorithms, which can significantly cut costs while maintaining service delivery.
“Locally manufactured TLD is a major step towards that goal,” WHO said, “but more action is needed.”
HIV – Key statistics according to WHO
- HIV remains a major global public health issue, having claimed an estimated 44.1 million lives to date. Transmission is ongoing in all countries globally.
- There were an estimated 40.8 million people living with HIV at the end of 2024, 65 percent of whom are in the WHO African Region.
- In 2024, an estimated 630,000 people died from HIV-related causes and an estimated 1.3 million people acquired HIV.
- There is no cure for HIV infection. However, with access to effective HIV prevention, diagnosis, treatment and care, including for opportunistic infections, HIV infection has become a manageable chronic health condition, enabling people living with HIV to lead long and healthy lives.
- WHO, the Global Fund and UNAIDS all have global HIV strategies that are aligned with the SDG target 3.3 of ending the HIV epidemic by 2030.
- By 2025, 95 per cent of all people living with HIV should have a diagnosis, 95 per cent of whom should be taking lifesaving antiretroviral treatment, and 95 per cent of people living with HIV on treatment should achieve a suppressed viral load for the benefit of the person’s health and for reducing onward HIV transmission. In 2024, these percentages were 87 per cent, 89 per cent, and 94 per cent respectively.
- In 2024, of all people living with HIV, 87 per cent knew their status, 77 per cent were receiving antiretroviral therapy and 73 per cent had suppressed viral loads.
Read also: Breaking the HIV bottleneck: Why sub-Saharan Africa must lead the way
Share this content:




Post Comment